2022 Reports 9 and 10 of the Auditor General of Canada to the Parliament of Canada
Report 9—COVID-19 Vaccines
At a Glance
Overall, the Public Health Agency of Canada and Health Canada, supported by Public Services and Procurement Canada, responded to the urgent nature of a rapidly evolving coronavirus pandemic by working together to obtain a sufficient number of COVID‑19 vaccine doses for provinces and territories to vaccinate everyone living in Canada. At the end of May 2022, the agency reported that about 82% of people who were eligible to be vaccinated at that time had received at least 2 doses. This was the largest mass vaccination program in Canadian history.
Although the government was successful in sending a sufficient number of doses to provinces and territories, the Public Health Agency of Canada ended up with a large surplus of doses. This led to vaccine wastage because some of the doses expired before they could be used or donated. At the end of May 2022, there were 32.5 million doses in inventory, and using unclassified and public documentation, we estimated those doses to be worth about $1 billion. The majority of those doses will expire by the end of 2022, resulting in more wastage if they are not used or donated soon. Canada, like other countries, is trying to donate some of its surplus. In addition, there were delays in implementing the information technology system that the Public Health Agency of Canada acquired to track the distribution and use of vaccine doses, necessitating workarounds for inventory management. Not all of the system’s functionalities were being used by the end of our audit.
Because of known problems that were not addressed before the pandemic, sharing case‑level details of COVID‑19 vaccine surveillance data was not as effective as it should have been. When the pandemic began in early 2020, the Public Health Agency of Canada did not have regulations or finalized agreements with the provinces and territories to clearly outline what public health surveillance information to share and how to share it. By the end of our audit period, the agency had not received permission from all provinces and territories to share detailed case‑level information on vaccine safety with Health Canada, vaccine companies, and the World Health Organization. Data sharing will continue to be important as new vaccines are developed and manufactured, including in Canada, to keep Canadians safe.
Why we did this audit
- Because factors such as global travel, urbanization, and climate change are making health emergencies like the COVID‑19 pandemic more likely to occur.
- Widespread immunization is one of the best tools for protecting people against infectious diseases. To reduce deaths, hospitalizations, and negative effects on Canadians’ health and social and economic well‑being during future health emergencies, it is important to learn from this pandemic and to be better prepared for future public health emergencies.
Our findings
- Public Services and Procurement Canada secured a sufficient supply of vaccine doses for Canada.
- The Public Health Agency of Canada equitably allocated vaccine doses to provinces and territories and distributed requested doses in a timely way.
- The Public Health Agency of Canada was unsuccessful in its efforts to minimize vaccine wastage.
- Despite data‑sharing issues, the Public Health Agency of Canada and Health Canada responded to vaccine surveillance data.
Key facts and figures
- By 19 January 2021, Public Services and Procurement Canada had put in place 7 advance purchase agreements for up to 414 million potential doses.
- On 27 July 2021, the Government of Canada announced that Canada had received more than 66 million doses of COVID‑19 vaccines. This meant that there were enough doses (2 doses each) to fully vaccinate every person in Canada who was eligible at that time (people aged 12 years and older).
- There were close to 32.5 million doses in federal, provincial, and territorial inventories. By the end of our audit period, the majority of these remaining doses still had a shelf life and could be used in booster campaigns or donated; however, most will expire by the end of 2022 if unused.
Highlights of our recommendations
- To minimize further wastage, the Public Health Agency of Canada should draw on the lessons learned from its management of the COVID‑19 vaccine supply and work with other implicated federal organizations and stakeholders to adjust its management of COVID‑19 vaccine surpluses.
- The Public Health Agency of Canada, in collaboration with Health Canada and the provinces and territories, should resolve barriers to
- better share vaccine surveillance information among themselves
- provide access to the Canadian Adverse Events Following Immunization Surveillance System to Health Canada
- provide surveillance data, including case‑level details as needed, to the World Health Organization and vaccine companies in a timely manner
Please see the full report to read our complete findings, analysis, recommendations and the audited organizations’ responses.


International vaccine donations support Goal 3 of the United Nations’ Sustainable Development Goals, which includes “vaccines for all” as part of one of its targets. The Government of Canada committed to donating the equivalent of 200 million COVID‑19 vaccine doses internationally by the end of 2022. This number includes 50.6 million doses that the agency deemed surplus. We found that as at 31 May 2022, only 15.3 million of these 50.6 million doses had been donated. We also found that, despite a commitment to donate doses with as much shelf life left as possible (ideally 14 to 16 weeks), other countries accepted multiple donations that left them only from 4 to 8 weeks to administer the vaccines before they expired. Officials told us that there are challenges with donations that are outside of Canada’s control, including the willingness of other countries to accept donations offered. Furthermore, many countries are now trying to donate their surplus doses, resulting in a slowdown in demand for donations.
Visit our Sustainable Development page to learn more about sustainable development and the Office of the Auditor General of CanadaOAG.
Exhibit highlights
Most unused doses in Canada will expire by the end of 2022
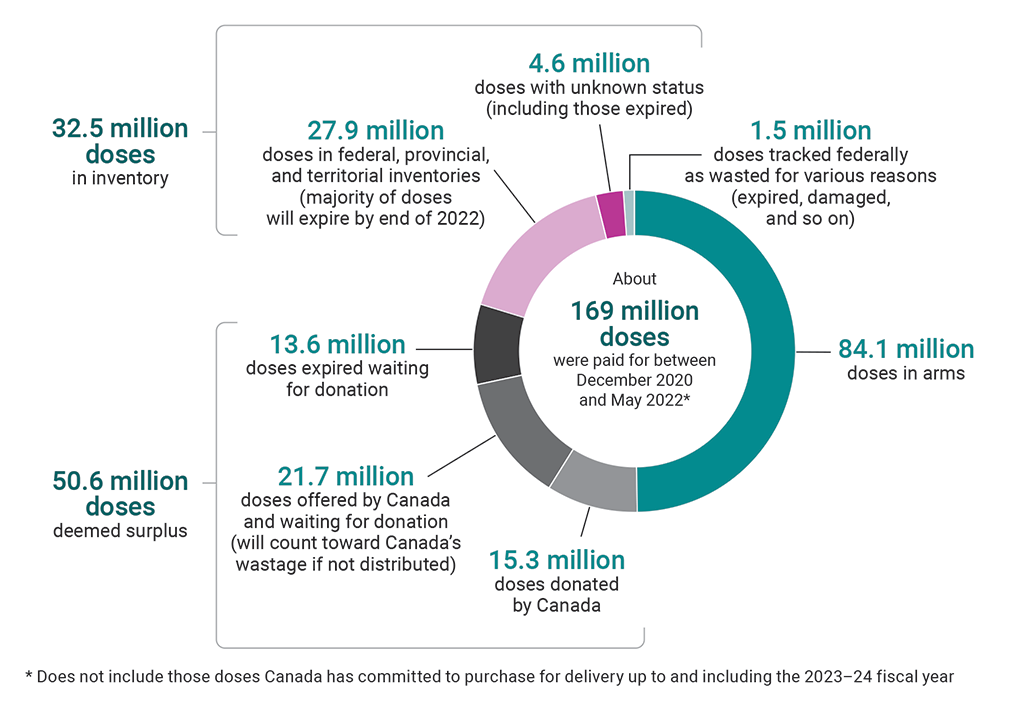
Source: Public Health Agency of Canada
Text version
This pie chart shows the breakdown of the about 169 million vaccine doses that were paid for between December 2020 and May 2022. This number does not include those doses Canada has committed to purchase for delivery up to and including the 2023–24 fiscal year.
About half of the 169 million doses were doses in arms. The remaining doses consisted of the following:
- There were 32.5 million doses in inventory. Of these, 27.9 million were doses in federal, provincial, and territorial inventories (the majority of these doses will expire by the end of 2022), and 4.6 million were doses with unknown status (including those expired).
- There were 50.6 million doses deemed surplus. Of these, 15.3 million were doses donated by Canada, 21.7 million were doses offered by Canada and waiting for donation (these will count toward Canada’s wastage if not distributed), and 13.6 million were doses expired waiting for donation.
- There were 1.5 million doses tracked federally as wasted for various reasons (expired, damaged, and so on).
Infographic
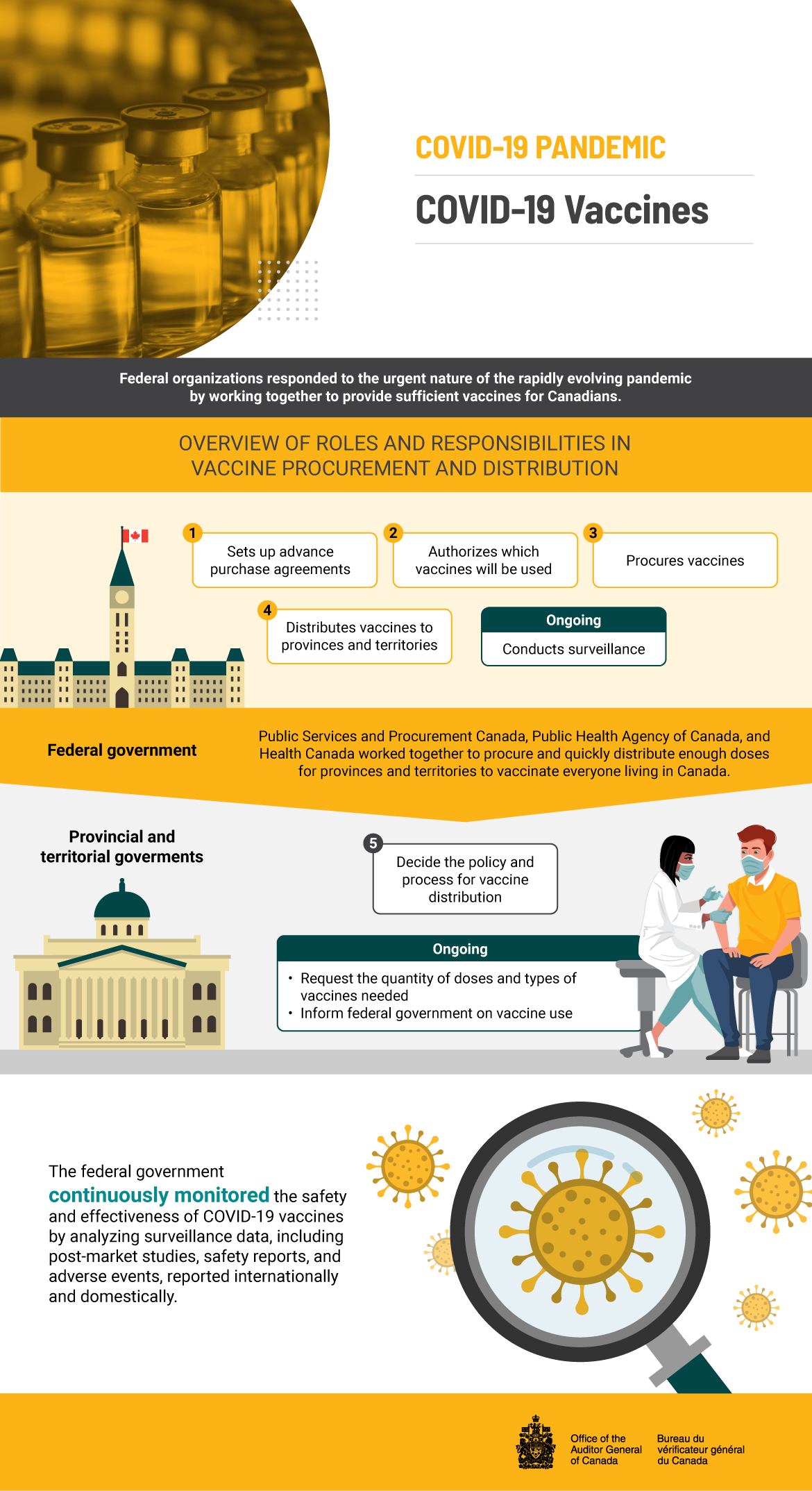
Text version
COVID‑19 Vaccines
Federal organizations responded to the urgent nature of the rapidly evolving pandemic by working together to provide sufficient vaccines for Canadians.
Public Services and Procurement Canada, Public Health Agency of Canada, and Health Canada worked together to procure and quickly distribute enough doses for provinces and territories to vaccinate everyone living in Canada.
Overview of roles and responsibilities in vaccine procurement and distribution:
Step 1—The federal government sets up advance purchase agreements.
Step 2—The federal government authorizes which vaccines will be used.
Step 3—The federal government procures vaccines.
Step 4—The federal government distributes vaccines to provinces and territories.
The federal government is also responsible for conducting ongoing surveillance.
Step 5—Provincial and territorial governments decide the policy and process for vaccine distribution.
Provincial and territorial governments also have the following ongoing responsibilities:
- Request the quantity of doses and types of vaccines needed.
- Inform the federal government on vaccine use.
The federal government continuously monitored the safety and effectiveness of COVID‑19 vaccines by analyzing surveillance data, including post‑market studies, safety reports, and adverse events, reported internationally and domestically.
Related information
Entities
Tabling date
- 6 December 2022
Related audits
- 2021 Reports of the Auditor General of Canada to the Parliament of Canada
Report 8—Pandemic Preparedness, Surveillance, and Border Control Measures
